What causes marine battery fires?
- Insights
- 14 September 2022
Lithium-ion batteries has, since the technology reached the market in the early 90’s, made large scale electrification possible. Everything from cellphones, laptops to e-scooters, e-bikes, cars, trucks, and vessels now depend on lithium-ion batteries for power and energy.
But apart from all its qualities, there is one challenge. Battery fires.
As lithium-ion batteries are being used everywhere, headlines about fires in buses, cars and even e-bikes are becoming more common. And unfortunately, vessels are no exception to this development. This is not an industry secret, it’s just not a topic that is discussed openly.
But we should. Safety should be top priority for all operators and shipowners, and to build safe vessels we need to understand the root cause of why batteries sometimes behave like this, and how to mitigate the risks.
In this article, we take a maritime perspective on battery safety, outlining:
- Why it needs to be prioritized
- The basics of battery safety
- What causes battery fires
- Implications of battery safety
Why is marine battery safety prioritized?
All fires are bad. But in a marine application, battery fires can have catastrophic outcomes. In fact, even more so than in a car or e-bike, just because you are isolated out at sea and rescue operations are far more complex. In addition, a large vessel will carry the equivalent of ten, twenty or even more EV-batteries on board. That means that if a fire occurs, the material available to fuel the fire is 10, 20 or 100 times that of a car.
At the same time, batteries are the best option to electrify a vast range of maritime applications in terms of range, weight, cost (TCO) and life cycle demands. Batteries are an important part of maritime electrification; we just need to build safe applications and make sure that we do what we can to minimize the risks involved.
Battery safety basics
There is a need to better understand what risks are involved, what causes marine battery fires, and what can be done to prevent them. In this article we will discuss the basics. Feel free to contact us if you want to a deeper technical understanding of marine battery safety.
The foundation of all batteries is what is commonly referred to as the cell chemistry. It’s the composition of materials used in the cathode (positive electrode) and anode (negative electrode), electrolyte, and the isolation in between that make up the core of every single battery.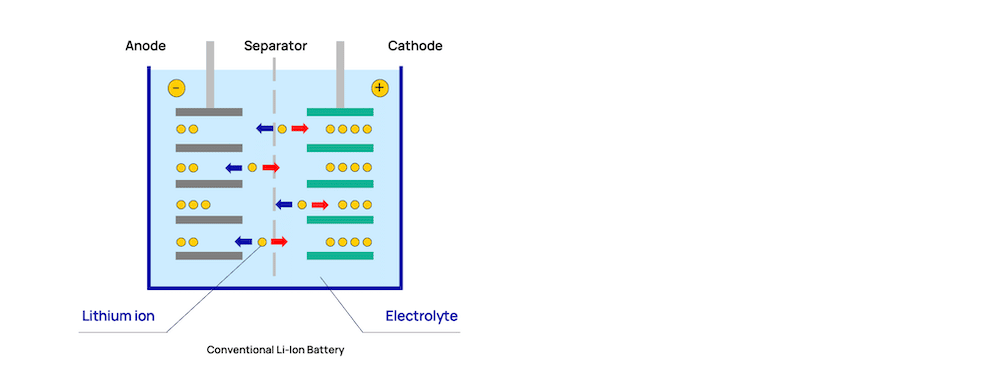 In the maritime world, the most common lithium-ion chemistries used are NMC, LFP and LTO. All of these are lithium-ion batteries but have variations in their composition to give them varying characteristics and qualities. NMC are the clear dominant and is also the chemistry used in the majority of EVs, e-bikes and scooters.
In the maritime world, the most common lithium-ion chemistries used are NMC, LFP and LTO. All of these are lithium-ion batteries but have variations in their composition to give them varying characteristics and qualities. NMC are the clear dominant and is also the chemistry used in the majority of EVs, e-bikes and scooters.
When we discuss battery safety, we make a distinction between three types of safety approaches:
- safety monitoring, battery management systems (BMS) etc.
- fire suppression systems, gas vents, sprinklers etc.
- inherent safety, cell level safety
All means to increase the level of safety are needed to ensure system stability and resistance to external and internal threats.
We need monitoring to make sure that we oversee any change in the system and can react to that accordingly.
We need systems that reduce the effects of fires and suppress them if they happen. And battery compartments, ventilation and isolation must follow strict class rules to make sure external factors cannot impact battery modules and cells.
But obviously, if a battery is inherently safe – i.e., have a close to zero risk of erupting fires or explosions, that is the best way to steer clear of catastrophic events.
To better understand why this is the case, we need to understand what causes battery fires.
What causes battery fires?
Combustion engines that run on petrol or diesel rarely self-combust, or spontaneously go off in flames. All fires are caused by accidents, or mechanical failures in the engine. For batteries, both these scenarios are possible. Just like a combustion engine, batteries can be damaged by accidents and external abuse but unlike them, lithium-ion batteries can erupt seemingly out of the blue. It doesn’t necessarily occur when the battery is in use or being charged.
This table shows that many possible failure events in batteries can be easily detected, controlled, and protected against. Like overcharging for instance, can be detected with voltage and current monitoring, and shut off at the event.
Failure modes for lithium-ion batteries
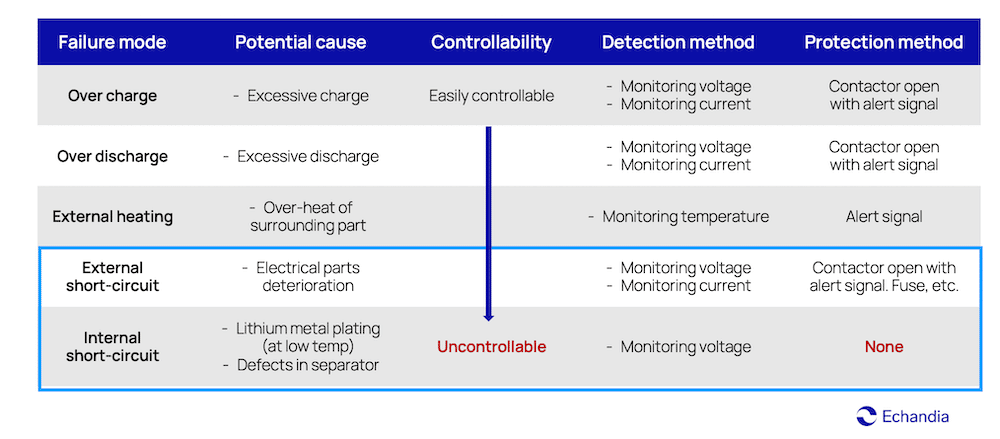
But there’s one thing that is almost impossible to control and protect against, and that is internal short-circuiting which will make the battery go into a state of thermal runaway.
Whatever the root cause is, the thermal runaway event looks the same. Increasing cell temperature will cause so called exothermic reactions, which in turn propagate through the hundreds and thousands of cells in the battery system. It’s a vicious circle that will happen faster than anyone has the chance to react upon.
Inherent battery safety will save lives
As we saw, most failure modes are controllable and can be protected against.
But as history teaches us over and over again, we can’t always be certain that external factors won’t happen. There are basically always risks that accidents and mishaps will happen in and around the battery compartment, that can lead to serious failures.
In essence, we can’t fully control external factors, but we can make a choice to lower the probability of fires occurring all together as an effect of them. Inherent safety is a safety net for the unforeseen events. Magnus Eriksson, CSO – Echandia
Leaning on our safety precautions such as monitoring, and suppression systems is fundamental, and obviously mandatory. But in the event of an unforeseen event, you still need a safety net. Using an inherently safe battery means making a choice to lower the probability of catastrophic events when an external event occurs.
Why is LTO inherently safe?
There are two characteristics of the LTO chemistry that gives the LTO battery its defining safety qualities.
- No dendrite build-up and no risk for ruptured isolator
- self-healing mechanism that prevents short circuit in case of penetration
What are dendrites?
Of the root causes for internal short-circuit, dendrite build-up is the most sinister and complex, because it grows slowly over time in the battery cell and can’t be detected.
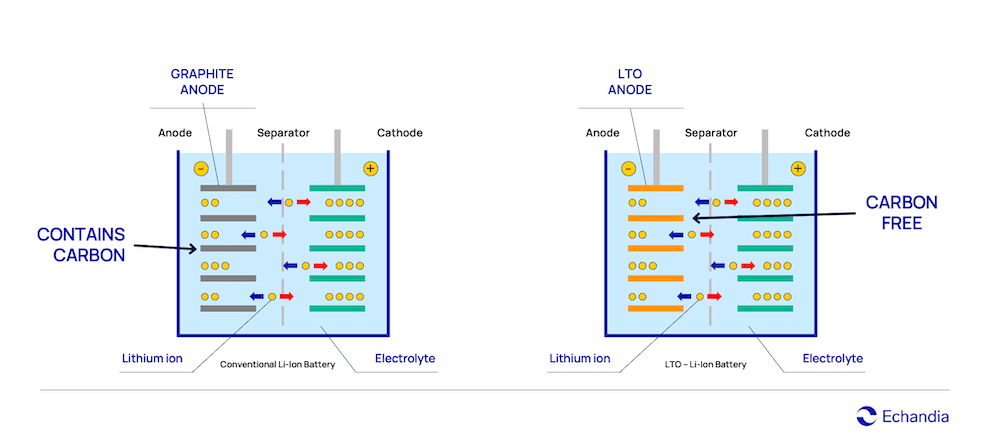
Dendrite build up is one of the major challenges for lithium-ion batteries and the cause for undetectable internal short-circuits. LTO is the only battery composition that do not have problem with dendrite build up and the reason is in its chemical composition. LTO uses lithium titanium oxide (LTO) in its anode while conventional lithium-ion batteries usually have graphite anodes containing carbon.
Dendrites are a branch-shaped metallic lithium, that are formed when being exposed to overvoltage (for example when charging quickly or charging in cold temperatures) and is deposited on the graphite anode. When dendrites grow, they eventually reach the cathode by piercing through the thin material separating the anode and the cathode (called separator), and the battery short circuits.
If an unlikely event happens – it won’t burn
We now understand better why LTO wont easily short-circuit. One more important thing to understand about the inherently safe LTO-battery is that if an external or internal event where to happen, it won’t behave in the same way as traditional lithium-ion battery with a graphite anode.
In a carbon-based anode, a short-circuit event, will cause the voltage to drop rapidly and the temperature rise very quickly. Typically, in a LTO anode, voltage and temperature are almost unaffected and rises only slightly long after the carbon-based battery cell have erupted.
LTO also has a self-protection mechanism, which makes it resistant to extreme abuse, such as being penetrated by foreign objects. If some external material gets in between the cathode and anode, the lithium ions are instantly released from the LTO close to the short circuit point which turns the LTO anode into an insulator (high-resistive phase). This stops the flow of excessive short circuit currents and will mitigate thermal runaway.
These batteries can take a lot of abuse and that is crucial in maritime since they are used in harsh environments. You can almost do anything to it, run a nail through it and it won’t go into thermal runaway, while another chemistry will explode. There’s no surprise that these types of batteries (LTO) are being used in naval applications. Magnus Eriksson, CEO – Echandia
The implication of these qualities is that no matter what unlikely event occurs, the LTO battery will not cause catastrophic events.
Maritime battery safety implications
We are in the process of electrifying the maritime industry and we depend on reliable and safe batteries to make that happen. The amount of energy stored, and the sheer number of vessels being electrified is increasing rapidly the responsibility for all stakeholders has increased.
Increasing the level of safety in batteries will be beneficial for commuters, travelers, and global trade.
An implication less talked about is that battery safety will become an insurance liability with expanding premiums for risk compositions and system configurations. It is very likely that as insurance companies start to better understand maritime battery safety issues, qualities that reduce the risk of internal short-circuiting will be preferred.
Monitoring and suppression technology is getting more advanced, and the industry is constantly learning and improving in these areas. By better understanding the role of battery chemistry we can significantly lower the probability of catastrophic events.
External events occur randomly and unpredictably. Building on LTO is one way to make sure that fires don’t.