Why is LTO an inherently safe battery?
- Insights
- 22 September 2022
All batteries have varying characteristics, which is why evaluating them needs to be done with operational demands at hand. When dimensioning and choosing battery system, we value certain parameters such as energy density, power capabilities, system size and weight. The optimal solution, depends on outlook, priorities and the operational profile at hand.
However, the need for real safety, durability under rough conditions and for extended periods, should always be prioritized.
Battery safety in the maritime world is a key issue that needs to be better understood by all stakeholders. We need industry wide collaboration as well as a better understanding of what constitutes a safe system and how to handle the risk of unforeseen events.
When we discuss battery safety in maritime settings, we make a distinction between three types of safety approaches:
- Monitoring, detection, and prevention methods
- Fire suppression systems, gas vents, sprinklers etc.
- Cell level safety, i.e. – inherent safety features of the battery
All the measures we can take to increase the level of safety are important and necessary to ensure system stability and resistance to external and internal threats.
With the battery management system (BMS) we detect and monitor the state of the battery system and can react to that any change in it, accordingly. This typically includes voltage level, current and temperature.
In a similar fashion, fire suppression, gas and air ventilation as well as extinguishing systems are needed if an unlikely event occur, and we need to react to that.
Ultimately, though it is the chemical composition and the design of the cell that determines the theoretical performance of a complete system as well as its propensity to internal short circuits and thermal runaway situations.
The role of the cell chemistry for safety
Aside from external safety precautions, such as monitoring, supervision, fire suppression systems and fire extinguishing – we rely on the battery cells internal, inherent qualities. Variations in chemistry, composition and design is what gives the battery system its fundamental, inherent safety features.
In previous articles we have described the basic safety aspects of marine battery systems. In this article we specifically focus on the LTO (lithium-titanium-oxide) cell chemistry that is being used in demanding heavy-duty applications, due to its differentiating qualities.
All batteries are built on the same basic structure. They have a positive cathode, negative anode, electrolyte, and a thin separator between them. Typically, what differs between the types of available batteries are variations in the cathode and anode materials.
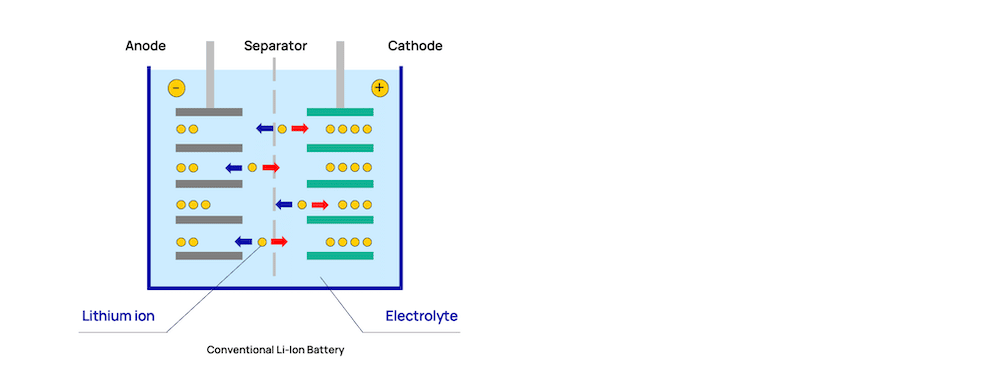
The LTO battery – the maritime workhorse
LTO has historically been misunderstood as heavy and not as energy dense as competing chemistries. However, it more than make up for its lower energy density per kilogram when it comes to building a complete system.
Its capability to discharge more of its available energy (depth of discharge – DOD) over a long period of time, means that there is less need for oversizing, or carrying buffer energy to last over the vessels design life. Which in turn mean size and weight can still be lower, compared on a system level.
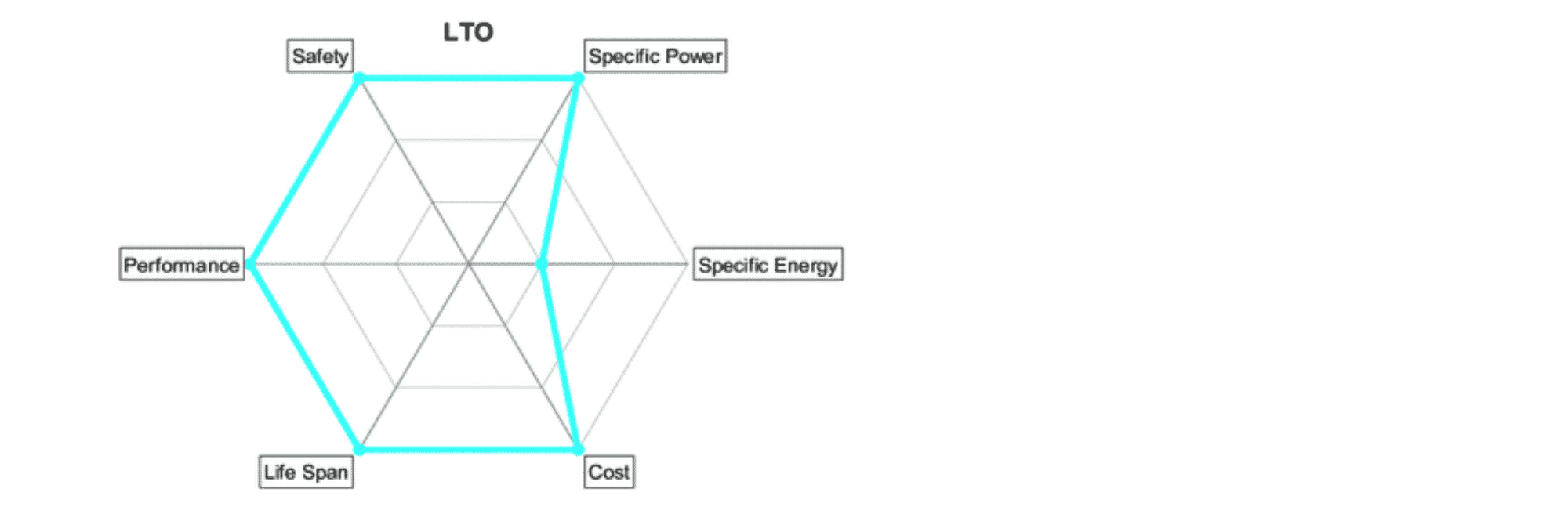
All batteries are tradeoffs between the same six characteristics, defined with this spider chart. LTO boasts superior qualities in safety, power, performance, and life span. Cost is usually higher per kWh, but lower on system level due to better life span and less need for oversizing.
But why is it safer than conventional lithium-ion batteries?
LTO batteries contain lithium-titanium-oxide as anode material instead of graphite. The use of titanium improves the cycle life of the battery and has a better temperature performance, compared to other lithium-ion counterparts
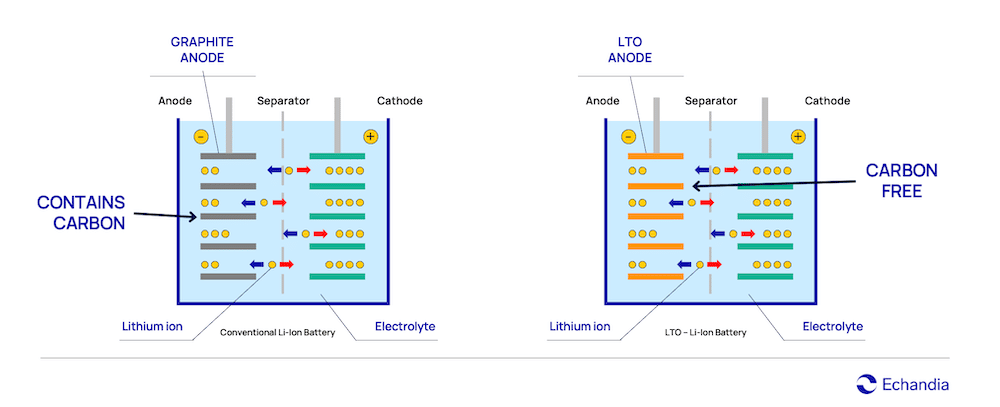
By using lithium-titanium-oxide in the anode, we achieve a number of positive characteristics, which apart from making it possible to build compact and durable systems, also have implications for battery safety. These are:
- No dendrite build-up and no risk for ruptured isolator
- Almost zero swelling during charge-discharge (no volume changes)
- Rapid charging and low-temperature performance
- Self-healing mechanism that prevents short circuit in case of penetration
Let’s go through them all.
No dendrite build-up on the anode
Dendrite build up is one of the major problems for lithium-ion batteries and is also the causes for undetectable internal short-circuits. This is a sinister and complex battery degradation problem, because it grows slowly over time within the battery cell and can’t be detected.
Unlike NMC, LTO do not have problem with dendrite build up. This is because of its anode material (lithium-titanium-oxide), that doesn’t contain carbon. Conventional lithium-ion batteries usually have graphite anodes containing carbon and are more prone to dendrite build-up over time.
Dendrites are a branch-shaped metallic lithium, that are formed when the anode is exposed to overvoltage (when potential drops to 0 V vs Li/Li+). With conventional lithium-ion batteries that uses carbon in the anode, lithium-ion insertion (when lithium ions get absorbed into a solid) happens when the potential is close to 0.1 V vs Li/Li+. So even just a slight overvoltage (0 V vs Li/Li+ compared to 0,1 V vs Li/Li+) increases the likelihood of dendrite deposits. This exposure can happen when charging rapidly or charging in cold temperatures for example.
The likelihood of this happening in an LTO anode is very low. In LTO anodes, lithium-ion insertion happens when the electric potential is at 1.5 V vs Li/Li+, (compared to 0 V vs Li/Li+). This provides a comforting margin.
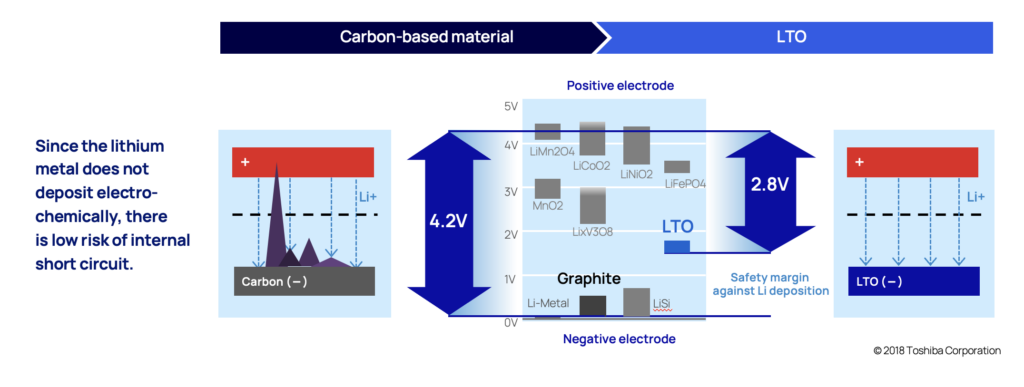
The slow continuous dendrite build-up also causes battery cells to degrade quicker than desired which is also the primary reason most battery chemistries won’t last as many cycles as LTO.
As dendrites grow, they eventually reach the cathode by piercing through the thin material separating the anode and the cathode (called separator), and the battery short circuits.
Minimal volume changes during charge and discharge
Almost all lithium-ion batteries expand and decompress as charge goes in vs out from the battery – i.e., absorbing and releasing lithium ions.
All batteries experience some level of physical volume changes when they are charged or discharged (i.e., absorbing or releasing lithium ions). This physical volume change, depending on how dramatic it is, can damage the battery’s material and will over time, lower its capacity and shorten its lifespan and potential cycle life.
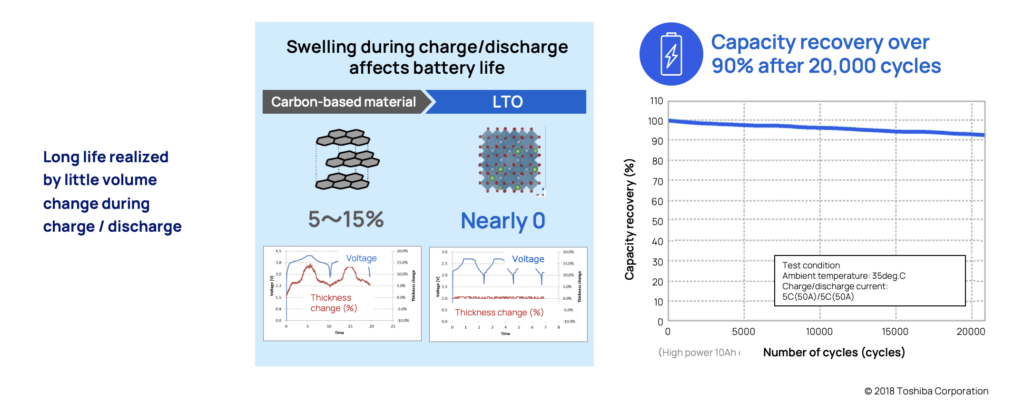
Many common chemistries can experience volume change of around 5-15 percent. LTO which are structured in what’s called a spinel shape, make them very stable and experience virtually zero change in volume. This is also one of the primary reasons the battery has such a long life span.
To verify this, tests have been performed on LTO batteries under extreme circumstances. After more than 20,000 cycles in 35°, the battery still held over 90% of its capacity.
Rapid charging and low-temperature performance
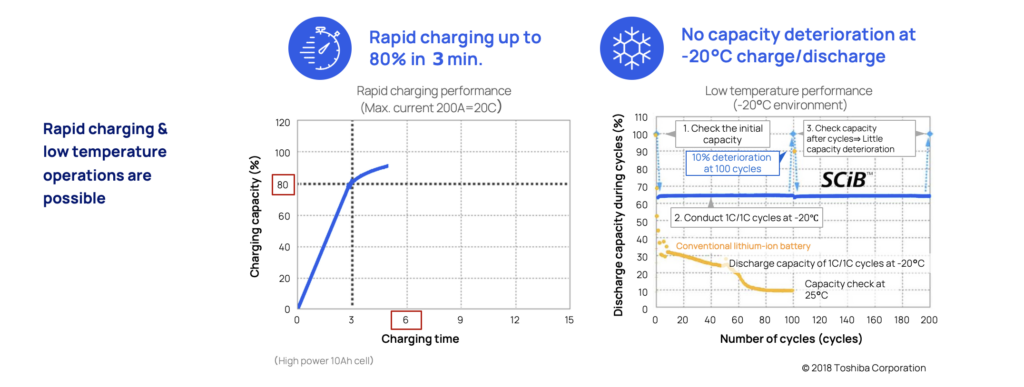
The LTO battery is perfect when there’s a need for safe and super fast charging. In three minutes (3) it can be charged up to 80 percent without causing dendrite build up that eventually will degrade the battery and increase the risk for internal short circuits.
The unique anode material is also resilient to extreme temperatures. In as low as -20° there is still no sign of dendrite build up or degradation. This makes LTO optimal for extreme maritime applications such as naval vessels, or commuter traffic in varying climates such as the Nordics and in hot and humid climate zones.
Self-healing mechanism that prevents short circuit in case of penetration
Short circuits in batteries often leads to catastrophic events, with a rapid thermal runaway event as the most likely outcome. One of the key advantages of LTO is that is has a self-protection mechanism against the dramatic effects of short-circuits.
External threats to battery systems are something to be cautious of. In maritime settings, humidity, and saltwater poses as a general risk for batteries, and for naval vessels a stray bullet or force towards the battery cells can lead to fires and explosions.
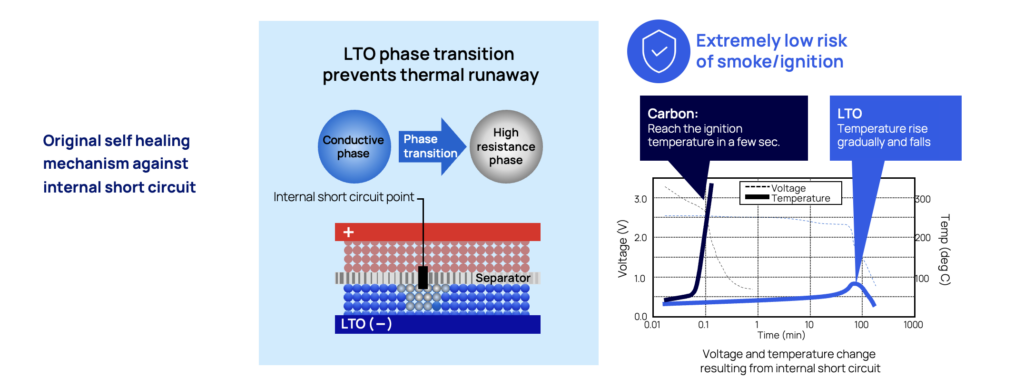 If this would happen – seeing that an object gets in between the cathode and anode by accident, the lithium ions are released from the anode (the LTO part) adjacent to the point of short circuit and will immediately turn the LTO anode into an insulator. By reacting like this, the initial thermal runaway will be controlled and stopped by preventing the flow of short circuit currents.
If this would happen – seeing that an object gets in between the cathode and anode by accident, the lithium ions are released from the anode (the LTO part) adjacent to the point of short circuit and will immediately turn the LTO anode into an insulator. By reacting like this, the initial thermal runaway will be controlled and stopped by preventing the flow of short circuit currents.
Summary
Echandia has chosen to build our battery system on LTO because of its qualities and characteristics, compared to conventional lithium-ion cells. Especially in maritime and heavy-duty applications, and risk environments – it has proven to be, compared on system level, both smaller, lighter and more cost efficient over time.
Visit our reference page, to better understand Echandia LTO in an application, like how we power a ship handling tugboat with batteries. Or how the city of Copenhagen safely transports commuters by water.
For more technical details, see our products page and technical documentation.